Titanium Dioxide – Pigment Chemistry
|
Listen to this Article::
|
GTranslate]
Table of Contents
The Role of Titanium Dioxide in Tattoo Pigments
Have you taken a look at the labels of your tattoo pigment lately? One thing you may notice is that there is almost always titanium dioxide in your pigments. Why is that?
Whether you know it or not, titanium dioxide (TiO2) is ubiquitous in our society. We find TiO2 in sunscreens, as a food additive, in most makeup products, and commercial products like paint and steel. It gets spread on our faces, doughnuts, and spaceships. When I said ubiquitous… I meant it.
Where Does Titanium Dioxide Come From?
TiO2 is sourced from raw minerals and is defined for specific uses by the size and dimension of the individual particles. The particles have a high refractive index, are stable in high UV applications, and are in demand globally, although costly to manufacture.
So why do we put this into our tattoo pigments? Is it safe? What should we know to be better-educated clients/tattoo artists globally?
TiO2 manufacturing is complicated and, since this is a website about tattooing better, we won’t get into what goes into the sourcing and processing. If you want to learn more, Google is your friend.
We will talk about some potential health consequences of TiO2, what knowledge is available about its safety and efficacy, and a few things about what these fancy particles do once they are in the skin.
Using TiO2 to Brighten Tattoo Pigments.
TiO2 is used in our tattoo pigments because it makes our pigments look brighter. This particle refracts light and disperses wavelengths that enhance colors (white light). The ability of this particle to absorb UV radiation is dependent on its particle size and can be tailor-made by manufacturers to refract the light at a specific wavelength.
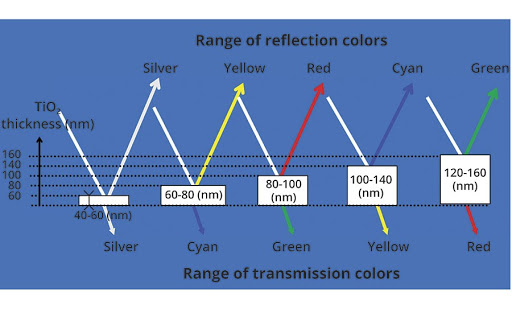
The Size of Pigment Particle Matters.
The light amplifying effects of TiO2 are well seen when you add even a small amount of TiO2 into pigments. When light hits the pigments, it bounces off and gets caught by our eyes, yet not all of the light bounces off the pigments directly towards the viewer. Most visible light reflects in any direction, painting anything it hits with the wavelengths of light that the pigment particle did not absorb.
With light coming into TiO2 being refracted (not reflected), the speed of the light hitting the particle changes even if the wavelength stays the same. That light can speed up or slow down, which changes the viewable light spectrum from what had initially hit the TiO2 particle. That energy is turned (like, literally) and forced to be normalized.
The ability of a substance to do this is measured on the refractive index, where 1 is the refractory index of a vacuum. The refractivity of TiO2 is around 2.5, which is nearly as high as a substance like a diamond which we know makes beautiful rainbows when hit with daylight!
What does that mean? Think of TiO2 as a filter, but instead of eating up all the available light (like a black pigment does), the light that enters a substance like TiO2 collects into like color spectrums. These focus spectrums, in turn, bounce (or bend through) a pigment already introduced to the skin or a painting. This focused light makes the pigments look more vibrant.
This additive can (more or less) puke out extra “shininess” and makes the amount of light hitting an object more efficient.
Titanium Dioxide Health Concerns
While TiO2 may be a food-safe product, there are questions about the safety when injected into the body. Here are a few papers that discuss the health effects of TiO2:
-
Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice.
-
Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts.
-
Human safety review of “nano” titanium dioxide and zinc oxide
-
Penetration of Nanoparticles through Intact and Compromised Skin
-
Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo
-
Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated Diatomites
…And a couple of papers for whoever wants to read them:
Titanium Dioxide white paper from DuPont
If you wish to skip the additional reading, here are some bullet points about the safety concerns with TiO2:
- TiO2 is classified as a type 2B carcinogen (possibly carcinogenic) and has been flagged by the French government as genotoxic, limiting its use in most things commercially available.
- How the particles are dispersed affects the product’s efficacy but can also affect the health consequences when particle sizes are taken into account.
- TiO2 is excellent at breaking down organic substances, especially those that degrade quickly when interacting with UV light. This possibly becomes a problem when organic pigments are used in tattooing.
- Damage to a cell’s DNA is almost always ubiquitous when TiO2 is introduced to a system, tissue, or organ (apart from the epidermis). Yet, more studies need to be done to fully understand the health consequences of things like sunscreen and cosmetics.
- TiO2 is a known irritant and using it has been shown to increase the chances of various skin ailments, like contact dermatitis.
- TiO2 can find its way into a body when introduced below the stratum basal.
My Takeaway from These Findings
We can assume TiO2 is safe in the short term when used in tattoo pigments. My main worry is that pigments mixed with TiO2 will degrade faster than those that do not contain them. This worry becomes even more significant when I think about the potential health consequences of degrading organic pigments, which the JRC Reports as a possible mega-negative.
A few arguments I can make against the possible adverse effects, or those effects which we see in-vitro that may not occur in-vivo, would be:
- We cannot assume the same health consequences from inhaled pigments compared to what is injected into the skin.
- The long-term consequences of organic pigments and products like TiO2 cannot be tested in the laboratory due to ethical concerns. If the results turn out that most who receive tattoos experience no adverse effects, the worry will have been for nothing (I hope)
- Pathways known to degrade pigments may be mitigated by the skin’s ability to produce melanin. If melanin-producing cells in the body can decrease the potential health consequences of tattoo pigments, only those most susceptible to the effects of light (those very light-toned) should avoid getting a tattoo.
- TiO2 being used in its normalized-scale-particle size (not nanoparticle sized) seems safer (cause less immediate health consequences) than nanoparticle sizes. If this is consistent, the nanoparticle dispersed pigments should be avoided.
That is about all I can think of today, so if anyone has something to add or wants to toss another article my way, feel free to reach out via email.




